The evolution of diversity of plant natural compounds
Project leader: Dr. Tobias Köllner
Terpenes are an ideal system to study structural diversity of natural compounds because of the large numbers of terpenes found in nature and their straightforward biosynthesis from activated five-carbon units. We are interested in the enzyme mechanisms that have evolved to produce these compounds and would like to utilize this knowledge for the rational design of novel enzymes and terpene products.
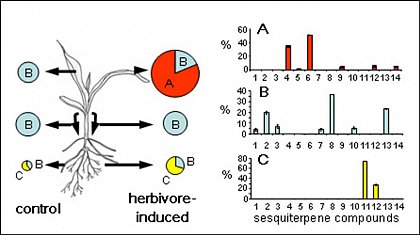
The sesquiterpene hydrocarbons of a maize seedling form three differentially regulated groups of which group A is induced by herbivory, B is ubiquitous and C is only found in the roots. The compounds are: 1: unknown, 2: α-copaene, 3: (E)-β-caryophyllene, 4: (E)-α-bergamotene, 5: sesquisabinene A, 6: (E)-β-farnesene, 7: unknown, 8: germacrene D, 9: zingiberene, 10: α-muurolene, 11: unknown, 12: β-bisabolene, 13: δ-cadinene, 14: β-sesquiphellandrene.
The enzyme class responsible for most of the diversification are the terpene synthases which are encoded in large gene families. The comparison of terpene synthase structure-function relationships is therefore an excellent tool to study evolutionary processes. We identified the terpene synthase gene family from maize and characterized their activity by heterologous expression, gas chromatography with isotopic tracers, mass spectrometry and nuclear magnetic resonance.
The unique catalytic mechanism of terpene synthases enables these enzymes to form multiple products from one substrate. To elucidate the reaction mechanism, we study structure-function relationships within the active site of the enzyme. Comparisons of closely related enzymes and directed alterations of single amino acids by in vitro mutagenesis helped us to identify structures responsible for the formation of multiple products. We identified several amino acids in the active center of the enzyme that determine product specificity and identified two catalytic pockets that appear to catalyze partial steps of the reaction. The goal of this study is to understand these fascinating reaction mechanisms and to engineer terpene synthases with a specific terpene spectrum. Furthermore, the phylogenetic analysis of structure function-relationships within maize and closely related grasses will indicate which evolutionary processes have shaped this gene family and yield to an understanding of its function.
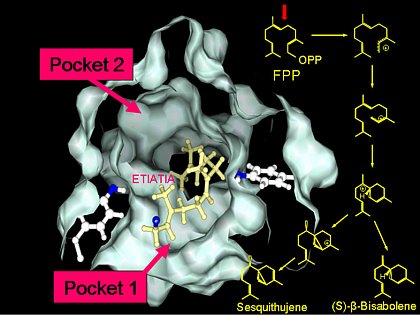
Model of the maize terpene synthase TPS4 active site with the proposed positions of the (E,E)-farnesyl diphosphate substrate. The proposed reaction pathway of the two main products of TPS4, 7-epi-sesquithujene and (S)-β-bisabolene is shown on the right. The reaction pockets 1 and 2 appear to control different steps of the reaction sequence.

