Starch biosynthesis: Engineering a monocot pathway into dicots
Reza Chamansara, Manish L. Raorane
Starch is a primary energy reserve in plants. As a staple food for humans and livestock, it also finds critical applications in the pharmaceutical and food industries, with annual production reaching approximately 4 billion tons. However, the efficiency of starch production varies significantly between monocots and dicots. In monocots, the cytosolic location of ADP-glucose pyrophosphorylase (AGPase) and the presence of a nucleotide sugar transporter (NST1) lead to a more energy-efficient starch synthesis pathway. Dicots, on the other hand, rely on a plastidial AGPase and ATP import. Moreover, the concomitant production of pyrophosphate by AGPase, while recoverable as NTP in the cytosol, is hydrolyzed to inorganic phosphate (Pi) in the plastid, incurring an energy loss of -21 kJ/mol PPi, resulting in lower efficiency.
This project proposes to enhance dicot starch biosynthesis by introducing the cAGPase and NST1 genes from monocots. These genes have been shown to confer a metabolic advantage in starch production. Leveraging recent advancements in biotechnology and crop engineering, this project aims to enhance dicot starch biosynthesis by introducing these monocot genes, thereby mimicking the more efficient monocot pathway and addressing the growing global demand for sustainable and efficient food production.
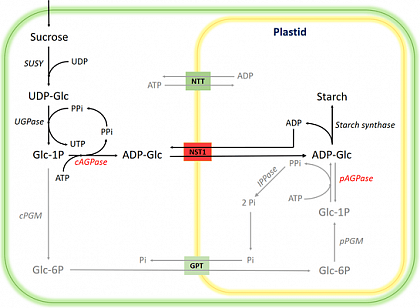
Pathway from sucrose to starch. The enzyme "cAGPase" and the transporter "NST1" have been introduced.




